Enzyme Technology: Application and Commercial Production of Enzymes
Introduction
Enzymes are the biocatalysts that are synthesized within the
bodies of living organisms. The enzymes are actually complex proteins and are
responsible for carrying out reactions that are related to life. Enzymes can
catalyse functions even when they are isolated from the cells i.e. in vitro.
Enzymes are basically biodegradable and nontoxic. Certain microorganisms can
produce large amount of enzymes for industrial applications.
 |
| Enzyme technology |
Enzyme technology
Enzyme technology consists of production, isolation,
purification, and utilization of enzymes for the welfare of mankind. Enzyme
technology also involves the production of more useful and efficient enzymes
through protein engineering and DNA recombinant technology.
The industry of biotechnology involved the commercial
manufacturing and utilization of enzymes as its major part. The specialized
fields like chemistry, biochemistry, process engineering and microbiology have
major contribution for the growth and development of enzyme technology.
General Considerations of Enzyme Technology:
Generally the techniques used for microbial production of enzyme
are similar to the methods employed for the production of other industrial
products. The main features are briefly described below.
1. Organisms selection
2. Medium formulation
3. Production method
4. Enzyme recovery and purification
An outline of the flow chart for enzyme manufacture by microorganisms
is depicted in Fig.1.
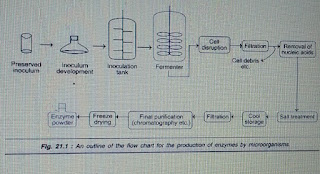 |
| Fig.1. An outline for production of enzymes by microorganisms |
Organism selection:
The microorganisms which are selected for enzyme production
should be capable of producing maximum quantities of required enzyme in a short
span of time and production of other metabolites should be minimum. After
selection of organism, the next step is strain improvement for optimization of
enzyme production by appropriate method such as UV production or mutagens. The
inoculum can be synthesized from the chosen organism in a liquid medium.
Medium formulation:
The culture medium selected should carry all the nutrient that
are essential for the sufficient growth of microorganism and eventually result
in large quantities of enzyme production. The ingredients used for medium
should have low cost and nutritionally intact. The frequently employed
substrates for the medium are molasses, corn steep liquor, starch hydrolysate,
whey, yeast extract and soy bean meal. Some pulses such as peanut and some
cereals such as wheat have also been employed. For maximum microbial growth and
good enzyme production, the pH of the medium should be optimum.
Production process:
Industrial manufacturing of enzymes is preferably accomplished by
submerged liquid conditions and to a smaller extent by solid-substrate
fermentation.The submerged culture technique gives more yield and contain
lesser chances of infection. Therefore this is the mostly used method. However,
solid substrate fermentation has its own historical importance and still in use
for the manufacture of fungal enzyme such as celluloses, pectinases, amylases
and proteases.
The batch or continuous sterilization techniques can be employed
for sterilization of medium. The start of the fermentation involves the
inoculation of the medium. The growth conditions such as pH, oxygen supply,
temperature and nutrient addition are regulated at optimal level. Anti-foam
agents can be used to minimize froth formation.
The fermentation process which is commonly employed for enzyme
production is batch fermentation and continuous process is employed to a lesser
extent. The fermentation process should contain sterile bio-reactor throughout
the process. The fermentation process has variable duration around 2-7 days in
majority of production processes. Various other metabolites are also produced
in addition to desired enzyme production. Therefore recovery and purification
of enzyme is necessary.
Recovery and purification of enzymes:
The desired enzyme to be manufactured may be present within the
cell that is intracellular enzyme or may be excreted inside the culture medium
that is extracellular enzyme. It depends on the commercial requirement that the
enzyme may be highly purified or may be crude. Furthermore, it may be in the
liquid or solid form. The degree of purity desired and the nature of enzyme
decides the steps involved in the recovery and purification process.
Generally, the recovery of an extracellular enzyme from the
broth is simpler in comparison to an intracellular enzyme. Special techniques
are required for cell disruption in order to release intracellular enzyme.
Several physical means such as high pressure, sonication and glass beads can be
employed to break down the microbial cell. Lysozyme enzyme can be used to break
the bacterial cell. The enzyme β-glucanase is used for yeasts. But the
enzymatic methods are expensive.
The recovery and purification steps are same for both
extracellular and intracellular enzymes after the disruption and release of
intracellular enzyme. The main consideration is to reduce the loss of desired
enzyme activity.
Removal of cell debris:
Cell debris can be removed by centrifugation or filtration.
Removal of nucleic acids:
There may be interference of the nucleic acids during the
recovery and purification of enzymes. Their interference can be removed by
precipitation and by the addition of poly-cations such as streptomycin,
polyamines and polyethyleneimine.
Enzyme precipitation:
Enzymes can be precipitated by utilizing organic solvents such
as ethanol, isopropanol and acetone and by using ammonium salts such as
ammonium sulfate. Precipitation has advantage since the precipitated enzyme can
be dissolved in a minimal volume in order to concentrate the enzyme.
Liquid-liquid partition:
Further concentration of desired enzyme can be accomplished by
liquid-liquid extraction utilizing polyamine or polyethylene glycol.
Separation by chromatography:
The separation and purification of enzymes can be carried out by
various chromatographic techniques. These include size exclusion, hydrophobic
interaction, ion exchange, dye ligand and affinity chromatography. Among these
methods, ion exchange chromatography is the most frequently employed
chromatography for enzyme purification.
Drying and packing:
Drying can be used to obtain the concentrated form of enzyme.
Film evaporators or steam dryers can be used for this purpose. After drying,
the next step is packing and ultimately enzyme can be marketed. Ammonium
sulphate suspensions can be used to achieve the stability of certain enzymes.
All the enzymes which are used in medical treatments or food
must have purity of high grade and must fulfil the required specifications by
regulatory bodies. Toxic material and harmful microorganism must be totally
absent in these enzymes and enzymes should not cause allergic reactions.
General Considerations for Regulation
of Microbial Enzyme Production:
A maximal yield of microbial enzymes can be obtained by optimising
the fermentation conditions such as nutrients, O2, pH, temperature
etc.. For this aspect, a complete understanding of the genetic regulation of
enzyme production is needed. Some of the general features of microbial enzyme regulation
are briefly discussed below
Induction:
Various enzymes are inducible i.e. they are prepared only by
using inducers. These inducers may be the substrate such as sucrose,
galactosides, starch or intermediate such as fatty acid, xylobiose , phenyl
acetate. Some enzymes and their inducers are given in Table 1.1.
Enzyme
|
Inducer
|
Invertase
|
Sucrose
|
Amylase
|
Starch
|
Lipase
|
Fatty acid
|
ẞ-Galactosidase
|
Galactosides
|
Penicillin G
amidase
|
Phenylacetate
|
Xylanase
|
Xylobiose
|
Table.1. Examples of
inducible enzymes with the inducers
The inducer compounds are costly and their processing such as
sterilization and addition at specific time is fairly difficult. Recently,
effors are being made to produce mutants of microorganisms to eliminate the
dependency of inducers.
Feedback repression:
Enzyme synthesis is significantly influenced by the feedback
regulation of the end product. This happens due the accumulation of large
quantities of the end product. The production of feedback regulated enzymes on
large scale is quite difficult. To overcome this problem, mutants have been developed
that lack feedback repression.
Nutrient repression:
The native metabolism of microorganism is devised in such a way
to avoid the production of unnecessary enzymes. The production of undesired
enzymes can be inhibited by nutrient repression. The nutrients may be nitrogen,
carbon, sulphate or phosphate fertilizers in the growth medium. Nutrient
repression must be overcome to produce enzymes on large scale.
The classical example of nutrient repression is glucose
repression. The synthesis of enzymes that are needed for the metabolism of rest
of the compounds is inhibited by the presence of glucose. By feeding
carbohydrates to the medium, the glucose repression can be overcome. Mutants
have been made to resist the catabolite repression by glucose. For various
microorganisms, other carbon sources such as lactate, succinate, pyruvate and
citrate are also used as catabolite repressors.
Microorganisms have also shown nitrogen source repression.
Ammonium ions or amino acids may be responsible for this. Ammonium salts are
commonly used as a source of nitrogen repression because these are inexpensive.
Nitrogen repression can be overcome by developing mutants.
Microbial Enzyme Production by Genetic Engineering:
The functional products of genes are enzymes. Therefore,
improved productions of enzymes can be developed through genetic engineering.
During the past 15 years, the microbial production of commercial enzymes have
been increased considerably through advancement in genetic engineering. Desired
enzyme genes can be now transferred from one organism to the other. The relevant genes of commercial enzymes
having potential uses can be now cloned and insertion can be carried out into a
suitable production house.
Cloning strategies:
Cloning strategies involves developing the cDNA library for the
mRNA and creating the oligonucleotides probes for the selected enzymes. The
specific cDNA clones can be identified by hybridization with oligonucleotide
probes.
 |
| Fig.2. Cloning |
For the production of desired enzyme, the next step is
transformation of industrially significant host organism such as Aspergillus
oryzae. High quality industrial enzymes can be prepared by using this approach.
A couple of enzymes prepared by using cloning strategies are discussed below:
1. Fat strains in the fabrics can be removed effectively by the
enzyme lipolase present in fungus Humicola lanuinosa. However due to the low
level production, industrial synthesis of lipolase by this organism is not
possible. So the genes responsible for lipolase production have been isolated,
cloned and inserted into the Aspergillus oryzae.
Thus, industrial production of this enzyme was successfully
obtained. Lipolase shows stability and resists degradation by proteases that
are frequently used in detergents. Therefore lipolase is a strong candidate for
utilizing in fabric washing due to these properties.
2. Rennet (chymosin) is commonly used enzyme in making cheese.
It is mainly produced by the stomach of young calves. As a result, its supply
is short. This has been solved by cloning the genes of chymosin for its
industrial production.
Modification of industrial enzymes by protein engineering:
Protein engineering and site directed mutagenesis can be used to
alter the structure of enzymes. The changes in the enzymes have been carried to
achieve increased stability, resistance to oxidation, improved catalytic
function, high tolerance to organic solvents and alkali and changed substrate
preferences.
Site directed mutagenesis can be employed to change the selected
amino acids at specific positions for the production of enzymes with desired
properties. Phospholipase A2 that resist high concentration of acid
have been structurally modified by genetic engineering. The modified enzymes
are more efficient for using as food emulsifier. Industrial production of
enzymes with desired properties have been achieved by genetic engineering in a
cost effective manner.




No comments:
Post a Comment